Selected Publications
Kellen Moulton, Nicholas B. Morrill, Adam M. Konneker, Brian D. Jensen, Richard R. Vanfleet, David D. Allred, and Robert C. Davis
This paper examines the effect of iron catalyst thickness on the straightness of growth of carbon nanotubes (CNTs) for microelectromechanical systems fabricated using the CNT-templated-microfabrication (CNT-M) process. SEM images of samples grown using various iron catalyst thicknesses show that both straight sidewalls and good edge definition are achieved using an iron thickness between 7 and 8 nm. Below this thickness, individual CNTs are well aligned, but the sidewalls of CNT forests formed into posts and long walls are not always straight. Above this thickness, the CNT forest sidewalls are relatively straight, but edge definition is poor, with significantly increased sidewall roughness. The proximity of a device or feature to other regions of iron catalyst also affects CNT growth. By using an iron catalyst thickness appropriate for straight growth, and by adding borders of iron around features or devices, a designer can greatly improve straightness of growth for CNT-MEMS.

Crystalline films and isolated particles of vanadium dioxide (VO2) were obtained through solid phase crystallization of amorphous vanadium oxide thin films sputtered on silicon dioxide. Electron back-scattered diffraction (EBSD) was used to study the crystals obtained in the thin films, to differentiate them from different vanadium oxide stoichiometries that may have formed during the annealing process, and to study their phase and orientation. EBSD showed that the crystallization process yielded crystalline vanadium dioxide thin films, semi-continuous thin films, and films of isolated particles, and did not show evidence of other vanadium oxide stoichiometries present. Indexing of the crystals for the orientation study was performed using EBSD patterns for the tetragonal phase of vanadium dioxide, since it was observed that EBSD patterns for the monoclinic and tetragonal phases of vanadium dioxide are not distinguishable by computer automated indexing. Using the EBSD patterns for the tetragonal phase of vanadium dioxide, orientation maps showed that all VO2 crystals that were measurable (approximately the thickness of the film) had a preferred orientation with the c-axis of the tetragonal phase parallel to the plane of the specimen. (C) 2011 Elsevier B.V. All rights reserved.

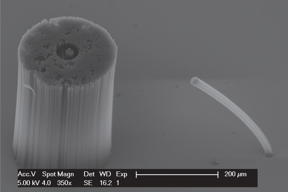
Walter C. Fazio, Jason M. Lund, Taylor S. Wood, Brian D. Jensen, Robert C. Davis, and Richard R. Vanfleet
Carbon nanotubes can be grown vertically from a substrate to form dense forests hundreds of microns tall. The space between the nanotubes can then be filled with carbon using chemical vapor deposition to create solid structures. These infiltrated structures can be detached from the substrate and operated as single-piece MEMS. To facilitate the design of compliant microdevices using this process, we explored the influence of two fabrication parameters—iron layer thickness and infiltration time—on the material’s mechanical properties, using the fracture strain to judge suitability for compliance. We prepared samples of a simple meso-scale cantilever beam pattern at various levels of these parameters, applied vertical loads to the tips of the beams, and recorded the forces and deflections at brittle failure. These data were then used in conjunction with a nonlinear FEA model of the beams to determine Young’s modulus and fracture stress for each experimental setting. From these data the fracture strains were obtained. The highest fracture strain observed was 2.48%, which is approximately 3.5 times that of polycrystalline silicon. This was obtained using an iron layer thickness of 10 nm and an infiltration time of 30 minutes. We used a test device—a compliant gripper mechanism for holding mammalian egg cells—to demonstrate the use of this material in compliant MEMS design.

Lei Pei, Jonathan Abbott, Kyle Zufelt, Andrew Davis, Richard Vanfleet, Matthew R. Linford, and Robert Davis (et al.)
Here we report a straightforward method for fabricating freely suspended, thin, carbon nanotube (CNT) membranes infiltrated with polymers. A CNT film was made by compressing (rolling) vertically aligned carbon nanotubes (VACNTs) on a silicon substrate. A nanotube-polymer composite film was then fabricated by spin casting a polymer layer on top of the flattened CNT film. The composite film was subsequently released from the silicon substrate by dipping in HF solution, resulting in thin, smooth, suspended membranes. To aid in releasing intact, quality films, a mesh frame was adhered to the films prior to release. Characterization of the film and membrane was performed via scanning electron microscopy (SEM) and atomic force microscopy (AFM). This process is a new approach for making thin, reinforced, smooth films or membranes with high concentrations of CNTs, which may lead to higher performance materials.

R. Vanfleet (et al.)
It is known that processing of silicon implanted with vanadium, Si:V, at high temperature-pressure, HT-HP, can lead to magnetic ordering within the V-enriched area. New data concerning structure of Si:V (prepared using V(+) doses, D = (1-5) x 10(15) cm(-2), and energy, E = 200 keV), as implanted and processed for up to 10 h at HT <= 1400 K under enhanced hydrostatic pressure, HP <= 1.1 GPa, are presented. In effect of implantation, amorphous (a-Si) area is produced near range of implanted species. Transmission electron microscopy, secondary ion mass spectrometry, X-ray, and synchrotron methods were used for sample characterisation. At HT-HP the a-Si layer is subjected to solid phase epitaxial re-growth. Depending on HP, distinct solid phase epitaxial re-growth and formation of VSi(2) are observed at HT >= 720 K. HP applied at processing results in the improved solid phase epitaxial re-growth in Si:V. This can be related, among others, to the effect of HP on diffusivity of V(+) and of implantation-induced point defects. Our results can be useful for development of the new family of diluted magnetic semiconductors.
